Answers
Explanation:
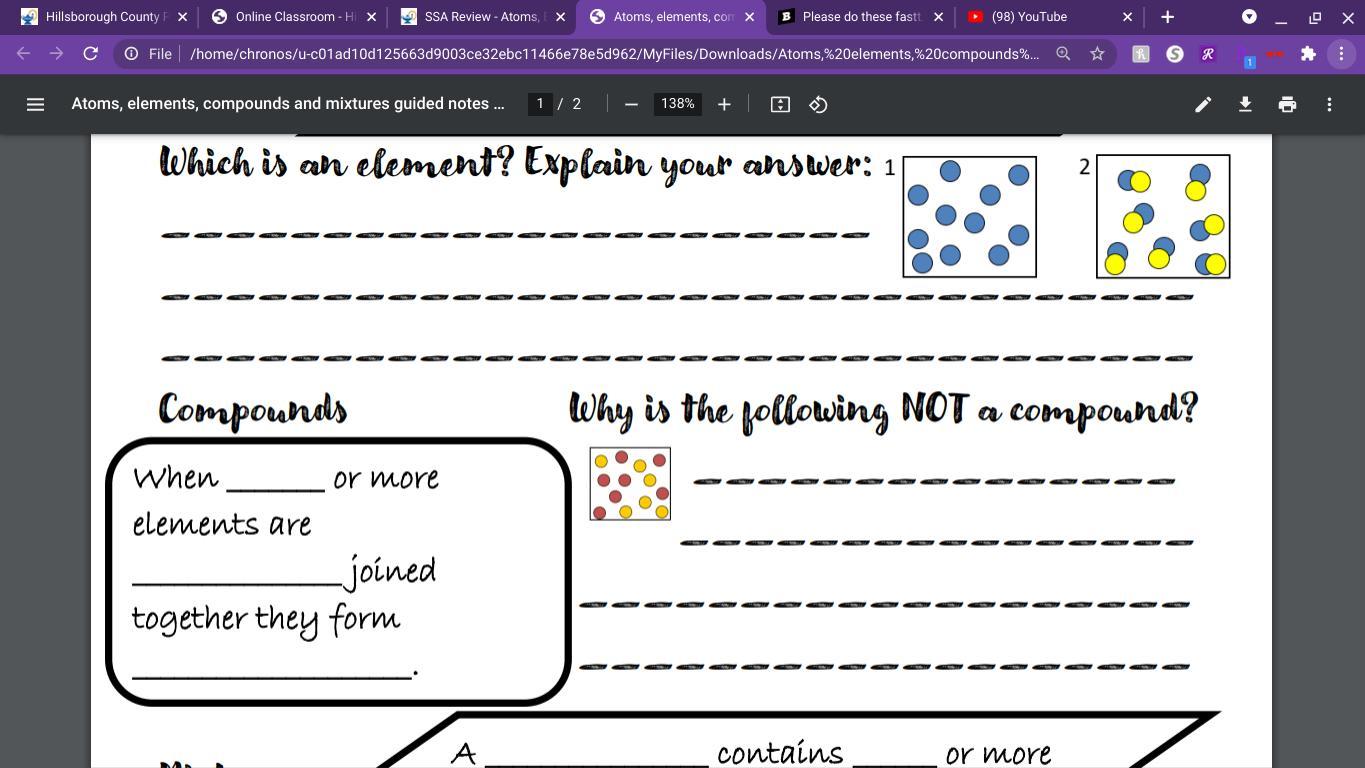
An element is define as a substance which contains only those atoms which have same number of protons. Basically, an element contains atoms of one type only.
For example, a piece of sodium metal will contain only atoms of sodium.
An element can never be divided further into simpler substances. So, in the given figure box 1 is containing atoms of only blue color is an element.
A compound is a substance formed by the chemical combination of two or more different atoms present in a fixed ratio.
For example, [tex]MgCl_{2}[/tex] is a compound which contains atoms of Mg and Cl in a 1:2 ratio.
The box containing pink and yellow balls in not a compound because here atoms are not chemically combined to each other but they are just present as individual atoms in same box. Hence, this is not a compound.
Therefore, when one or more elements are in a fixed ratio joined together they form a compound.
Related Questions
Using a Punnett square, what genotypes would you expect in the F2 offspring?
F1 generation
F2 (option A)
F2 (option B)
Father
Father
Father
A
A
A
A
A
a
Mother
Mother
Mother
a
Аа
«Аа
a
Аа
Аа
А
AA
Аа
a
Аа
Аа
a
Аа
Aai
aa
a
А
Answers
Answer:
can you answer mine i will help you! please
Explanation:
What can be different between the products and reactants in a balanced chemical equation?
Answers
Answer:Reactants are starting materials and are written on the left-hand side of the equation. Products are the end-result of the reaction and are written on the right-hand side of the equation.
Explanation:
The reactants in a balanced chemical reaction are the starting material which reacts together to give the products in by the regrouping of atoms in the reactants.
What is a balanced chemical equation?The balanced chemical equation of a reaction represents the perfect stoichiometry of each reactants and products with their states of matter in brackets.
In a balanced reaction, the number of each element in the reactant side must be equal to their number in the product side. The number of each elements and groups in a reaction can be balanced by multiplying with suitable integers.
The atoms in the reactants are regrouping to form the products. Thus, the identity of the new products is entirely different form that of reactants in the reaction.
To find more on balanced reactions, refer here:
https://brainly.com/question/14280002
#SPJ2
How much of a 60.0 gram sample of Carbon-14 DECAYED after 17,100 if 5
its half-life= 5,700 years? *
7.50 g
52.5 g
15.0 g
30.0 g
Answers
The mass of 60 g of carbon -14 remains after 17100 years is 7.50 g. Thus, option a is correct.
What is radioactive decay?The unstable isotopes of atoms undergo radioactive decay by the emission of charged particles. The radioactive decay is a first order reaction.
The rate constant k = 1/t ln (M0/Mt)
where, M0 and Mt be the initial mass and mass after t respectively.
Given the initial mass = 60 g
half life t1/2 = 5700 years.
decay constant k = 0.693 / t1/2
= 0.693 / 5700 = 0.00012 yr⁻¹
Apply the values in the first order rate constant equation as follows:
0.00012 yr⁻¹ = 1/ (17100 yrs) ln (60/Mt)
60/Mt = 7.996
Mt = 7.50 g.
Therefore, the mass of the mass of 60 g of carbon -14 remains after 17100 years would be 7.50 g.
To find more on radioactive decay, refer here:
https://brainly.com/question/1770619
#SPJ2
Calculate the number of moles found in 3.045x1024 atoms of helium.
PLS HELP
Answers
Explanation:
so for this u have to use this equation where
Moles = number of particle/6.02×10^23
= 3.045 × 10^24/6.02×10^23
= 5.0581
write it to 3 S.F so 5.06 moles
Bacteria live on skin of humans. The bacteria receive food and shelter form human beings. Most bacteria are harmless and pose little or no threat to humans. This is an example of
Answers
Answer: symbiotic relationship
Explanation:
Marie Curie's work with radioactive materials eventually lead to her death. At the age of 66, what
disease did Madame Curie die from?
Answers
Answer:
Radiation-induced Luekimia
Explanation:
They worked with many radioactive elements, so it caused Leukemia which is
a disease in which the bone marrow and other blood-forming organs produce increased numbers of immature or abnormal leukocytes.
Hoped this helped
Add coefficients to balance the equation to make water.
H2(g) +O2 (g)H20 (1)
Answers
Answer:
2H2+2O2=2H2O
Explanation:
hope this helps
why does solid silver, Ag, conducts electricity while solid silver chloride, AgCl, does not.
Answers
Answer: A solid silver, Ag, conducts electricity because it contains ions or electrons while solid silver chloride, AgCl, does not conduct electricity because AgCl does not dissociate to give ions or electrons.
Explanation:
Solid silver (Ag) is considered to be a good conductor of electricity because there are more number of free electrons or movable atoms are present in it.
As electricity is the flow of ions or electrons. So, more is the number of ions or electrons present in a substance more will be its conductivity.
Whereas solid silver chloride is a precipitate that is insoluble in water and therefore, it will not give ions or electrons.
As a result, silver chloride is not able to conduct electricity.
The [H+] is 0.001 M. What is the pH?
Answers
Answer: So the pH of the solution is 4.
DIRECTIONS: Underline the selected word item that best completes the statements.
1. (Climate, Weather) is an overall atmospheric condition of a place of 30 years
and more while (Climate, Weather) is a condition for a short period of time.
2. (Climate, Weather) is influenced by latitude, altitude, ocean current, and
topography.
3. The (higher, lower) the altitude, the (colder, warmer) the climate.
4. (Windward, Leeward) area of the mountain forms precipitation while (Windward,
Leeward) gives dry air and warm weather.
5. Ocean currents bring (cold, warm) water and rain from the equator to the
poles and (cold, warm) water from the poles toward the equator
Answers
Explanation:
(Climate, Weather) is an overall atmospheric condition of a place of 30 years and more while (Climate,Weather) is a condition for a short period of time.
2. (Climate, Weather) is influenced by latitude, altitude, ocean current, and topography.
3. The (higher, lower) the altitude, the (colder, warmer) the climate.
4. (Windward, Leeward) area of the mountain forms precipitation while (Windward,Leeward) gives dry air and warm weather.
5. Ocean currents bring (cold, warm) water and rain from the equator to the poles and (cold, warm) water from the poles toward the equator
What is the fixer solution equation?
Answers
Answer:
Fixation involves these chemical reactions (X = halide, typically Br−): AgX + 2 S2O32− → [Ag(S2O3)2]3− + X. AgX + 3 S2O32− → [Ag(S2O3)3]5− + X. In addition to thiosulphate the fixer typically contains mildly acidic compounds to adjust the pH and suppress trace amounts of the developer.
Water absorbs energy when it undergoes___
A. freezing
B. deposition
C. condensation
D. melting
Answers
Answer:
d. melting
As heat enters the water the particles move much faster, bumping into each other. Therefore, more heat means more energy!
Hopesthis helps!
How does renal regulation at the kidneys work in removing acid and restoring equilibrium
Answers
Answer: The kidneys help maintain the acid–base balance by excreting hydrogen ions into the urine and reabsorbing bicarbonate from the urine.
Explanation:
A 3.20-mol sample of gas occupies a volume of 350. mL at 300.0 K. Determine the
pressure of the gas.
Answers
Answer:
[tex]P \approx 225atm[/tex]
Explanation:
From the question we are told that:
Moles of sample n=3.20_mol
Volume V=350mL
Temperature T=300k
Generally the equation for ideal gas is mathematically given by
[tex]PV=nRT[/tex]
[tex]P=\frac{nRT}{V}[/tex]
[tex]P=\frac{3.20*0.08206*300}{350*10^{-3}}[/tex]
[tex]P=225.079atm[/tex]
[tex]P \approx 225atm[/tex]
HELPPPP ASAPP 10 POINTS
Answers
Write the molecular formula of poly(glycine)
Answers
Answer:
C2H5NO2
Explanation:
Glycine | C2H5NO2 - PubChem.
weight of glycine= 75.07 g/mol
Identify the limiting reactant when 2.20 g of calcium reacts with 4.5 g of chlorine gas to produce calcium chloride. Write a balanced equation before starting.
I"LL GIVE YOU 30 POINTS AND NAME YOU BRAINLIEST IF YOU ANSWER THIS FOR MEEEEE PLZZZZZZZZZZZZZZZZZZZZZZZZZZZZZZ ITS FOR MY STUDY GUIDE!!!!!!
Answers
Answer:
2.20*4.5=?? you have to solve the multiplication ok, or its not learning
Explanation:
Which two processes of the water cycle absorb energy from the Sun?
A evaporation and precipitation B sublimation and evaporation C condensation and transpiration D precipitation and condensation
Answers
Answer: of Earth's atmosphere occurs as energy, primarily from the sun, causes liquid water to transform to another phase. As this occurs, liquid water absorbs energy, causing it to evaporate and form water vapor. The process of evaporation absorbs tremendous amounts of incoming solar energy.
Explanation:
Una muestra de 0,830 g de MgCl2 se disuelve en 350 g de agua. Si la densidad de la disolución es 1,09 g/mL
Answers
Answer:
NMIKCORQW
J3WEFONCIONV WEJFQOMS;DX
A pacemaker:
A. sends electrical impulses to a prosthesis, causing it to move
through brain signals.
B. measures the distance traveled with a prosthetic leg.
C. tracks the speed traveled with a prosthetic leg and adjusts
movements accordingly.
D. sends electrical impulses to the heart, causing it to beat more
regularly
SUEMIT
Answers
Explanation: A pacemaker is implanted in the chest and helps to treat abnormal heart rhythms especially those causing your heart to skip beats or beat too slow.
proper significant figure: 27.01 + 14.369
Answers
Answer:
put the bigger number on top then addExplanation:
41.369
Which type of reaction does an element replace an ion in a compound, forming a new compound and new element?
a
Synthesis
b
Decomposition
c
Single Replacement
d
Double Replacement
e
Combustion
Answers
Answer:
The answer is c . Single Replacement
Explanation:
A single replacement reaction, sometimes called a single displacement reaction, is a reaction in which one element is substituted for another element in a compound.
How many moles of H2O are produced from 3 moles of oxygen? 2H2 + O2 --> 2H2O
Answers
Answer: 6 mol
Explanation:
From the equation, we know that for every mole of oxygen consumed, 2 moles of water are produced.
This means that if 3 moles of oxygen are consumed, 3(2) = 6 mol of water are produced.
How many units long is the circumference of a circle with diameter of 18 units?
Answers
Answer:
56.52 units
Explanation:
hope that helps :)
Does this help
A nebula is often made up of hydrogen and helium gases.
True
False
Answers
Answer:
Nebulae are made of dust and gases—mostly hydrogen and helium. ... Eventually, the clump of dust and gas gets so big that it collapses from its own gravity. The collapse causes the material at the center of the cloud to heat up-and this hot core is the beginning of a star.
Explanation:
Hope this helps
How many moles of gallium chlorate, Ga(ClO3)3, must react in order to produce 674 kJ of energy according to the following reaction? 2 Ga(ClO3)3 à 2 GaCl3 + 9 O2 DH = - 130.4 kJ
Answers
Answer:
10.3 mol Ga(ClO₃)₃
Explanation:
Let's consider the following balanced thermochemical equation.
2 Ga(ClO₃)₃(s) ⇒ 2 GaCl₃(s) + 9 O₂(s) ΔH = - 130.4 kJ
According to the balanced thermochemical equation, 130.4 kJ of heat are released when 2 moles of gallium chlorate react. The number of moles of gallium chlorate that must react to produce 674 kJ of energy is:
674 kJ × 2 mol Ga(ClO₃)₃/130.4 kJ = 10.3 mol Ga(ClO₃)₃
Thermochemical equations are chemically balanced equations that take into account both the energy change and the physical states of all reactants and products. Energy is a reactant in an endothermic process, whereas it is a product in an exothermic reaction. Here the moles of Ga(ClO₃)₃ is 10.3 mol.
Let's consider the following balanced thermochemical equation.
2Ga(ClO₃)₃(s) ⇒ 2GaCl₃(s) + 9O₂(s) ΔH = - 130.4 kJ
According to the balanced thermochemical equation, 130.4 kJ of heat is released when 2 moles of gallium chlorate react. The number of moles of gallium chlorate that must react to produce 674 kJ of energy is:
674 kJ × 2 mol Ga(ClO₃)₃ / 130.4 kJ = 10.3 mol Ga(ClO₃)₃
To know more about thermochemical equation, visit;
https://brainly.com/question/10384873
#SPJ6
Which combinations of particles form ionic bonds/compounds?
Answers
Answer:
Iconic bond is formed by cation and anion. A compound is made when atoms of two or more elements bond in a chemical reaction.
Explanation:
An atom has 23 protons and 24 neutrons. Write both its NAME and it's SYMBOL using isotope notation.
Answers
Answer:
Vanadium is the name and the symbol is V #23
Explanation:
Which of these is a polar molecule?
1. SH2
2. CO2
3. F2
Answers
En una botella hay 2x10 25 moléculas de vinagre puro ¿Cuántos mol y cuántos gramos de esta sustancia habrá en la botella?
Answers
Answer:
33.2 moles y 1994 gramos.
Explanation:
¡Hola!
En este caso, dado que es posible para nosotros relacionar moles con gramos por medio de la masa molar y moléculas con moles por medio del número de Avogadro, resulta factible para nosotros primero calcular las moles en las moléculas dadas y de esta manera luego calcular los gramos, teniendo en cuenta una masa molar de 60.05 g/mol ya que el vinagre se puede estudiar como ácido acético (CH3COOH):
[tex]2x10^{23}molec*\frac{1mol}{6.022x10^{23}molec}=33.2mol[/tex]
Seguidamente, calculamos los gramos:
[tex]33.2mol*\frac{60.05g}{1mol}=1994g[/tex]
¡Saludos!