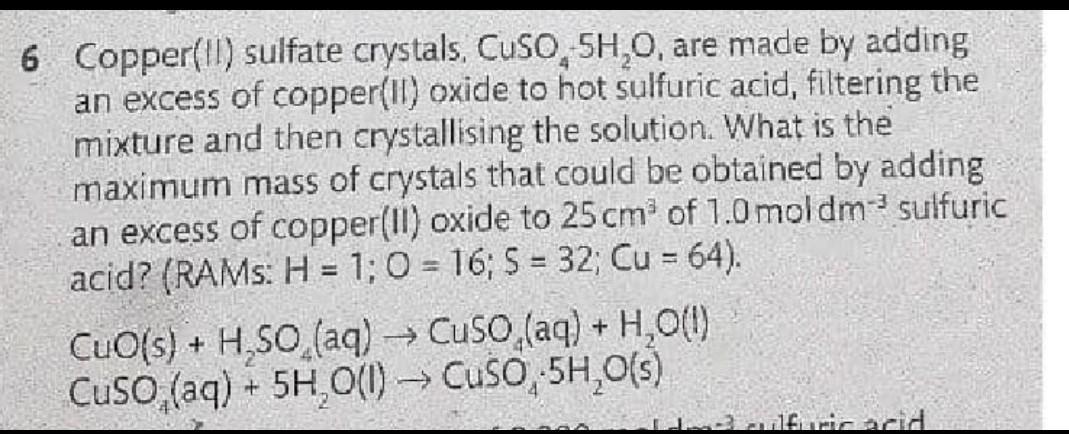Answers
Answer:
25cm³ = 0.025L of H2So4
Molarity of H2SO4 = 1moldm-³
Recall ... 1dm-³ = 1L
So the Molarity can also be 1mol/L
Mole = Molarity x volume in L
Mole of H2SO4 = 1mol/L x 0.025L
=0.025Moles of Sulphuric acid reacted.
From the equation of reaction
1mole of H2SO4 reacts to produce 1mole of Copper sulphate crystal
Since their Mole ratio is 1:1
It means that Since 0.025mole of H2SO4 reacted.... 0.025mole of CuSO4.5H2O would be produced
Nice.. OK
So we know the moles of CuSO4.5H2O produced
We can get the Mass
Recall
From
Mole=Mass/Molar Mass
Mass = Mole x Molar Mass
Molar Mass of CuSO4.5H2O = 64 + 32 + 16x4+ 5(2+16)
Mm= 250g/mol
Mass = 0.025mol x 250g/mol
= 6.25g of CuSO4.5H2O crystals Would be PRODUCED.
Related Questions
1:How many moles of ammonia will be produced if 12 moles of nitrogen are used?
2:How many moles of nitrogen are required to react with 6 moles of hydrogen?
3:Honors: If there are 40 moles of nitrogen and 30 moles of hydrogen, what is the limiting reactant? Explain your answer.
4:Honors: What is the mass of hydrogen required to produce 80 g of ammonia?
Please, I really need help, so I can graduate
Answers
Answer:
See Explanation
Explanation:
The reaction equation is
3H2(g) + N2(g) -----> 2NH3(g)
1)If 1 mole of N2 yields 2 moles of NH3
12 moles of N2 will yield 12 * 2/1 = 24 moles of NH3
2) 3 moles of nitrogen reacts with 1 mole of nitrogen
6 moles of hydrogen will react with 6 * 1/3 = 2 moles of nitrogen
3) The limiting reactant yields the least amount of product
If 1 mole of nitrogen yields 2 moles of NH3
40 moles of nitrogen yields 40 * 2/1 = 80 moles of NH3
If 3 moles of hydrogen yields 2 moles of NH3
30 moles of hydrogen yields 30 * 2/3 = 20 moles of NH3
Hence hydrogen is the limiting reactant
4) Number of moles in 80g of NH3 = 80g/17g/mol = 4.71 moles
3 moles of hydrogen produces 2 moles of ammonia
x moles of hydrogen produces 4.71 moles of ammonia
x = 3 * 4.71/2 = 7.065 moles of hydrogen
Mass = 7.065 moles of hydrogen * 2 g/mol
Mass = 14.13 g of hydrogen
PLEASE HELP, DUE AT 12:00
Answers
1. How many grams of sodium carbonate must be weighed out in order to make 2.5 kg of a 35.0%
(w/w) solution?
Answers
Answer:
... x M x V. • Example: Prepare 800 mL of 2 M sodium chloride. ... solutions are indicated by w/v% and are defined as the grams of solute per ...
15 pages·906 KB
240 g of water (specific heat = 4.186 J/g°C, initial temperature = 20°C) is mixed with an
unknown mass of iron (specific heat = 0.444 J/gºC, initial temperature 500°C). When
equilibrium is reached, the system has a temperature of 42°С. Find the mass of iron.
Answers
240 g of water (specific heat = 4.186 J/g°C, initial temperature = 20°C) is mixed with an unknown mass of iron (specific heat = 0.444 J/gºC, initial temperature 500°C). When equilibrium is reached,The answer for this would be 69.6
How do you find final temperature with specific heat?
You use q = mcΔT, but you assume aluminum = water and crack for This the final temperature.
We need to look up heat values (c) for aluminum and h20. This will uses 0.901 for aluminum and 4.18 use water
: (10)(130 - T)(0.901) = (200.0)(T - 25 (6)
Hence, The answer for this would be 69.6.
To learn more about specific heat click here:
https://brainly.com/question/1747943
#SPJ2
At what pressure would a 5.32 mol Cl2 sample occupy 5.08 L at 181.59 K? please help!!
Answers
Answer: At a pressure of 15.613 atm a 5.32 mol [tex]Cl_{2}[/tex] sample occupy 5.08 L at 181.59 K.
Explanation:
Given: Moles = 5.32 mol
Volume = 5.08 L
Temperature = 181.59 K
Formula used to calculate the pressure is as follows.
PV = nRT
where,
P = pressure
V = volume
n = no. of moles
R = gas constant = 0.0821 L atm/mol K
T = temperature
Substitute the values into above formula as follows.
[tex]PV = nRT\\P \times 5.08 L = 5.32 mol \times 0.0821 L atm/mol K \times 181.59 K\\P = \frac{5.32 mol \times 0.0821 L atm/mol K \times 181.59 K}{5.08 L}\\= 15.613 atm[/tex]
Thus, we can conclude that at a pressure of 15.613 atm a 5.32 mol [tex]Cl_{2}[/tex] sample occupy 5.08 L at 181.59 K.
g A closed-end manometer was attached to a vessel containing argon. The difference in the mercury levels in the two arms of the manometer was 9.60 cm. Atmospheric pressure was 783 mm Hg. The pressure of the argon in the container was ________ mm Hg. Selected Answer: Incorrect 773 Answers: 882 793 661 773 Correct 96.0
Answers
Answer:
Answer: 882mmHg
Explanation:
In a closed-end manometer, the gas added is under a pressure that is measured against atmospheric pressure. The difference in pressure between both gases is equal to the difference in mercury levels. Thus, the pressure of the argon can be:
783 mmHg - 96.0mmHg = 687mmHg
783 mmHg - 96.0mmHg = 879mmHg
Based on the options, the possible answer (The nearest to the second value) is:
Answer: 882mmHgWhat is the volume of nitrogen dioxide (in L) is produced from the complete reaction of 16.87g of lead (II) nitrate at STP?
Answers
Given the reaction N2 + 3H2 -->2NH3, what volume of hydrogen is necessary to react with five liters of nitrogen to produce ammonia, assuming constant temperature and pressure? How would you calculate the mass in grams of hydrogen chloride produced when 4.9 L of molecular hydrogen at STP reacts with an excess of chlorine gas.?
5
Which of the following is a factual statement about
hormones?
A They are the sole cause of mood swings
B
They cause boys' and girls' bodies to develop
C
They are a type of blood cell
D They are extremely difficult to deal with
Answers
Answer:
They cause boys' and girls' bodies to develop
Which image represents ether? Check all that apply.
Answers
Answer:
A) and D)
Explanation:
I just completed this assignment and these are the correct options!
In the given image, the compounds that represents ether are option 1 and 4, respectively.
Ether is a class of organic compounds that contain an oxygen atom bonded to two alkyl or aryl groups. The general formula for ethers is [tex]\rm R-O-R'[/tex], where R and R' are alkyl or aryl groups.
Compound 1 is 1-methoxypentane is an ether with the molecular formula [tex]\rm C_6H_{14}O[/tex]. It consists of a pentane chain (a straight chain of five carbon atoms) with a methoxy [tex]\rm (-OCH_3)[/tex] group attached to the first carbon atom. Compound 4 is methoxyethane. It is an ether with the chemical formula [tex]\rm C_3H_8O[/tex]. The structure of methoxyethane consists of a ethane chain (a straight chain of two carbon atoms) with a methoxy [tex]\rm (-OCH_3)[/tex] group attached to the first carbon atom.Therefore, compound 1 and compound 4 represents ether. The correct answer is option 1 and 4, respectively.
Learn more about Ether here:
https://brainly.com/question/28047849
#SPJ6
An electric kettle uses electrical energy to boil water. Energy from the electricity is transferred to the water, heating it up. An electric ice maker also uses electrical energy, but it freezes water to form ice. Since energy can’t be created or destroyed, and water loses potential energy when it freezes to form ice, what happens to the energy put into the ice maker and the energy released by the water?
Answers
Answer:
Since energy can’t be created or destroyed, and water loses potential energy when it freezes to form ice. The energy remains electrical but then changes to kinetic when Enough energy is released.
Explanation:
The excess electrical energy gained by water during ice forming process is removed as potential energy to maintain law of conservation of energy.
According to the principle of conservation of energy, energy can neither be created nor destroyed but can transformed from one form to energy.
The electrical energy used by the ice maker to freeze water works on the same principle of conservation of energy.
The electrical energy absorbed the water is used to lower the temperature of the water in order to form ice by removing heat from the water in form of potential energy. This happens to maintain the principle of conservation of energy.Thus, the excess electrical energy gained by water during ice forming process is removed as potential energy to maintain law of conservation of energy.
Learn more about conservation of energy here: https://brainly.com/question/166559
Which part of the graph represents how much energy the reactants need to gain to become products?
Answers
Answer: The answer is F
The answer is F , F shows the amount of energy the reactants need to gain to become products .
What are Potential Energy Diagrams ?
A potential energy diagram shows the change in potential energy of a system as reactants are converted into products.
From the figure attached we can understand the Eₐ is the activation energy reactants need to get converted into products.
Now when we study the figure( potential energy Diagram) given in the question ,
The reactants are energy level shown by G
The total energy required to get converted into products is denoted by H while ,
H - G is the actual energy required which is denoted by F.
Therefore F shows the amount of energy the reactants need to gain to become products .
To know more about Potential Diagram
https://brainly.com/question/12078312
#SPJ2
Help plz:)))I’ll mark u Brainliest
Answers
Answer:
82gS
Explanation:
2 Fe + 3 S → Fe2S3
↓ ↑
mol → mol
Fe S
[tex]\frac{95 g Fe }{} \frac{1 mol Fe }{55.85 g Fe } \frac{3 mol S }{2 mol Fe } \frac{32.07 g S}{1 mol S} = 82 g S[/tex]
pls help Which statement is true about the environment of urban areas?
Urban areas have higher temperatures.
Urban areas have few problems with soil erosion.
Urban areas have more rain infiltration into the soil.
Urban areas have less habitat fragmentation.
Answers
The statement that is correct about Urban areas is that they have higher temperatures.
Urban Area is a term to refer to cities. Urban areas are characterized by having a developed infrastructure (wide roads, vehicular bridges, wide platforms, tall buildings, residential areas, industrial areas, among others).
Recent studies affirm that urban areas are warmer than surrounding areas; This phenomenon is because the materials with are built buildings, roads, houses, and others, concentrate the sun's rays, increasing the temperature of cities. In addition, the lack of trees deepens this phenomenon, because the trees contribute to cooling by physicochemical processes such as evapotranspiration.
According to the above, it is possible to affirm that urban areas are hotter than their surrounding area because they lack vegetation, and the materials with which it is built contribute to the increase in temperature.
On the other hand, urban areas are characterized by habitat fragmentation, more problems with soil erosion, and less rain infiltration into the soil.
Learn more in: https://brainly.com/question/23587978
Answer:
A) higher temps
Explanation:
half equation for oxygen to oxide ions, aluminium ions to aluminium, magnesium to magnesium ions
Answers
g A chemical equilibrium exists when: A chemical equilibrium exists when: there are equal amounts of reactants and products. the rate at which reactants form products is the same as the rate at which products form reactants. the sum of reactant and product concentrations equals one mole. reactants are completely changed to products. the rate at which reactants form products becomes zero.
Answers
Answer:
the rate at which reactants form products is the same as the rate at which products form reactants
Explanation:
There is still a reaction happening just that the second one happens the opposite happens and keeps it at net 0
Which of the following compounds is most likely ionic?
Please help
Answers
Answer:
LiCl.
Explanation:
Hello there!
In this case, since it is known that the ionic compounds are specially formed between metals and nonmetals, it is possible for us to discard CO and PCl5 as they have both nonmetals.
On the other hand, since the bonds between nonmetals and transition metals like iron and copper may not be completely ionic due to the electronegativity trend, we infer that the most likely ionic compound is LiCl because of the large electronegativity difference.
Regards!
A simplified model of an electromagnet is shown here, where a loop of wire is placed around an iron nail. The wire is
connected to a battery. A student wants to test the strength of the electromagnet by measuring how many paperclips
the electromagnet can pick up. In order to increase the strength of the electromagnet, some modifications to the
apparatus can be made. Which questions are most relevant to increasing the strength of the electromagnet? Select
ALL that apply.
A)
Will wetting the nail increase the number of paperclips that can
be picked up?
B)
Does insulating the nail increase the number of paperclips that
can be picked up?
C)
Does increasing the current in the battery increase the number of
paperclips that can be picked up?
D)
Will increasing the number of loops of wire around the nail
increase the number of paperclips that can be picked up?
E)
Will positioning the loops of wire closer together on the nail
increase the number of paperclips that can be picked up?
Forces in Nature
Answers
Answer:
CDE
Explanation:
If your on USA test perp
The questions are most relevant to increasing the strength of the electromagnet are Does increase the current in .....?, Will increasing the number of loops of wire around the.....? and Will positioning the loops of….? Therefore, option C, D and E are correct.
What are the properties of electromagnet ?The characteristics known as electromagnetic properties determine how quickly a substance will absorb or emit electromagnetic radiations.
We may be familiar with electromagnetic radiations such as radio waves, microwaves, ultraviolet rays, infrared rays, and visible light rays.
Transverse waves characterize electromagnetic radiation.They move across fluctuating magnetic and electric fields so that they are perpendicular to the wave's path of propagation and at right angles to one other.
Thus, option C, D and E are correct.
To learn more about the properties of electromagnet, follow the link;
https://brainly.com/question/15502579
#SPJ6
help!!!
1. How many grams of Ag+ are present in 2.24 grams of silver
sulfite?
grams Agt.
2. How many grams of silver sulfite contain 1.75 grams of Agt?
grams silver sulfite.
Answers
Answer:
1. 0.82 gram of Ag+
2. 4.79 g of Ag₂O₃S
Explanation:
From the given information:
Total amount of Ag₂O₃S = 2.24 grams
Atomic mass of Ag+ =107.86 g/mole
molar mass of Ag₂O₃S = 295.8 g/mole
∴
The mass of the Silver (Ag) in grams is:
[tex]= Total\ amount \ of \ Ag^+ \times \dfrac{107.86 \ g/mol}{295.8 \ g/mol}[/tex]
[tex]=2.24 \times \dfrac{107.86 \ g/mol}{295.8 \ g/mol}[/tex]
= 0.82 gram of Ag+
2.
Here, the total amount of Ag₂O₃S = unknown
Atomic mass of Ag+ = 107.86 g/mole
molar mass of Ag₂O₃S = 295.8 g/mole
amount of Ag+ = 1.75 g
∴
The mass of Ag₂O₃S = [tex]Total \ amount \ of \ Ag^+ \times \dfrac{295.8 \ g/mol}{107.86\ g/mol}[/tex]
[tex]=1.75 \times \dfrac{295.8 \ g/mol}{107.86\ g/mol}[/tex]
= 4.79 g of Ag₂O₃S
Scientist use models of the solar system to
1 avoid the use of incorrect data
2 prevent duplication of their ideals
3 help explain their ideas
4 make theories non-testable
Answers
Which of these equations represent reactions that could be used in constructing an electrochemical c
Check all that apply
CH. +202 C02 +2H,0
Cr + Cu? → Cr?++ Cu
2 Ag*+Fe + 2Ag +Fe2
CI+Ag → AgCI
NH, *H* NH,
RETRY
Answers
Answer:
Cr+Cu2 -> Cr2+Cu
2Ag+Fe->2Ag+Fe2
Explanation: I did the assessment
what type of sugar has the largest surface area
a) powered/icing
b) raw
c) cubed
d) granulated
Answers
Answer:
d.) granulated
Because the granulated sugar has a greater surface area.
HELP PLEASE Excluding the noble gas group, how does the number of valence electrons in an element influence it chemical reactivity?
Answers
Answer: A. Elements with intermediate numbers of valence electrons are the least reactive.
Explanation: I Quizzed
................................................................
Answers
Answer:
..............,.,.,.,,..,,.,.,
Using the Lewis dot structures of magnesium and oxygen, predict the ionic formula
Answers
Answer:
(edit: nvm I figured it out, here is the answer)
Explanation:
Ions exists bound together in ionic formulae by electrostatic attraction then the ionic formula exists MgO.
What is meant by Lewis dot structures?The diagrams known as Lewis structures, also referred to as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures, depict the interactions between the atoms in a molecule as well as any lone pairs of electrons that may be present.
A chemical compound comprehended as an ionic compound in chemistry exists one that contains ions bound together by the electrostatic forces named as ionic bonding. The molecule exists often neutral despite containing both positively and negatively charged ions, or cations and anions. Ions exists bound together in ionic formulae by electrostatic attraction.
Magnesium and oxygen:
Cation [tex]$=\mathrm{Mg}^{2+}$[/tex]
Anion [tex]$=\mathrm{O}^{2-}$[/tex]
Net charge = + 2 - 2 = 0
Formula = MgO
Therefore, the ionic formula exists MgO.
To learn more about ionic compound refer to:
https://brainly.com/question/2687188
#SPJ2
How to solve for K when given your anode and cathode equations and voltage
Answers
Answer:
See Explanation
Explanation:
In thermodynamics theory the Free Energy (ΔG) of a chemical system is described by the expression ΔG = ΔG° + RTlnQ. When chemical system is at equilibrium ΔG = 0. Substituting into the system expression gives ...
0 = ΔG° + RTlnKc, which rearranges to ΔG° = - RTlnKc. ΔG° in electrochemical terms gives ΔG° = - nFE°, where n = charge transfer, F = Faraday Constant = 96,500 amp·sec and E° = Standard Reduction Potential of the electrochemical system of interest.
Substituting into the ΔG° expression above gives
-nFE°(cell) = -RTlnKc => E°(cell) = (-RT/-nF)lnKc = (2.303·R·T/n·F)logKc
=> E°(cell) = (0.0592/n)logKc = E°(Reduction) - E°(Oxidation)
Application example:
Calculate the Kc value for a Zinc/Copper electrochemical cell.
Zn° => Zn⁺² + 2e⁻ ; E°(Zn) = -0.76 volt
Cu° => Cu⁺² + 2e⁻ ; E°(Cu) = 0.34 volt
By natural process, charge transfer occurs from the more negative reduction potential to the more positive reduction potential.
That is,
Zn° => Zn⁺² + 2e⁻ (Oxidation Rxn)
Cu⁺² + 2e⁻ => Cu° (Reduction Rxn)
E°(Zn/Cu) = (0.0592/n)logKc
= (0.0592/2)logKc = E°(Cu) - E°(Zn) = 0.34v - (-0.76v) = 1.10v
=> logKc = 2(1.10)/0.0592 = 37.2
=> Kc = 10³⁷°² = 1.45 x 10³⁷
According to the following reaction, how many grams of
hydrobromic acid are needed to form 24.7 grams of bromine?
hydrobromic acid (aq) —>hydrogen (g) + bromine (1)
…grams hydrobromic acid
Answers
Answer:
25.1 g
Explanation:
Step 1: Write the balanced decomposition reaction
2 HBr(aq) ⇒ H₂(g) + Br₂(l)
Step 2: Calculate the moles corresponding to 24.7 g of Br₂
The molar mass of Br₂ is 159.81 g/mol.
24.7 g × 1 mol/159.81 g = 0.155 mol
Step 3: Calculate the moles of HBr needed to produce 0.155 moles of Br₂
The molar ratio of HBr to Br₂ is 2:1. The moles of HBr needed are 2/1 × 0.155 mol = 0.310 mol.
Step 4: Calculate the mass corresponding to 0.310 moles of HBr
The molar mass of HBr is 80.91 g/mol.
0.310 mol × 80.91 g/mol = 25.1 g
If only 0.186 g of Ca(OH)2 dissolves in enough water to give 0.230 L of aqueous solution at a given temperature, what is the Ksp value for calcium hydroxide at this temperature?
Answers
Answer:
[tex]Ksp=5.20x10^{-6}[/tex]
Explanation:
Hello there!
In this case, according to the solubility equilibrium of calcium hydroxide:
[tex]Ca(OH)_2\rightleftharpoons Ca^{2+}+2OH^-[/tex]
Whereas the equilibrium expression is:
[tex]Ksp=[Ca^{2+}][OH^-]^2[/tex]
It is firstly necessary to calculate the molar solubility given the grams and volume of the dissolved solute:
[tex]s=\frac{0.186g/(74.09g/mol)}{0.230L}=0.0109M[/tex]
Now, according to the Ksp expression, we plug in s as the solubility to obtain:
[tex]Ksp=(s)(2s)^2\\\\Ksp=(0.109)(2*0.0109)^2\\\\Ksp=5.20x10^{-6}[/tex]
Regards!
According to the solution equilibrium,
[tex]Ca(OH)_2 \rightleftharpoons Ca^{2+}+2OH^-[/tex]Now,
The molar solubility will be:
→ [tex]s = \frac{\frac{0.186}{74.09} }{0.230}[/tex]
[tex]= 0.0109 \ M[/tex]
hence,
The Ksp value will be:
→ [tex]Ksp = (s)(2s)^2[/tex]
By substituting the values,
[tex]= (0.109)(2\times 0.0109)^2[/tex]
[tex]= 5.20\times 10^{-6}[/tex]
Thus the above approach is right.
Learn more about temperature here:
https://brainly.com/question/23773843
make a chemical reaction with molecules
Answers
a warm air is rising as cooler air es in to take its place. what is the name for that?
Answers
Answer:
convection
hope this helps
have a good day :)
Explanation:
Identify the type fo reaction below: ____ N2+__H2→ __NH3 synthesis or double replacement
Answers
Answer:
Synthesis or combination.
Explanation:
Hello there!
In this case, according to the given chart containing the types of reactions, it turns out possible for us infer this given reaction:
____ N2+__H2→ __NH3
Is synthesis or combination, because it follows the pattern A + B --> C. Moreover, it can be balanced as shown below:
N2+3H2→ 2NH3
Regards!