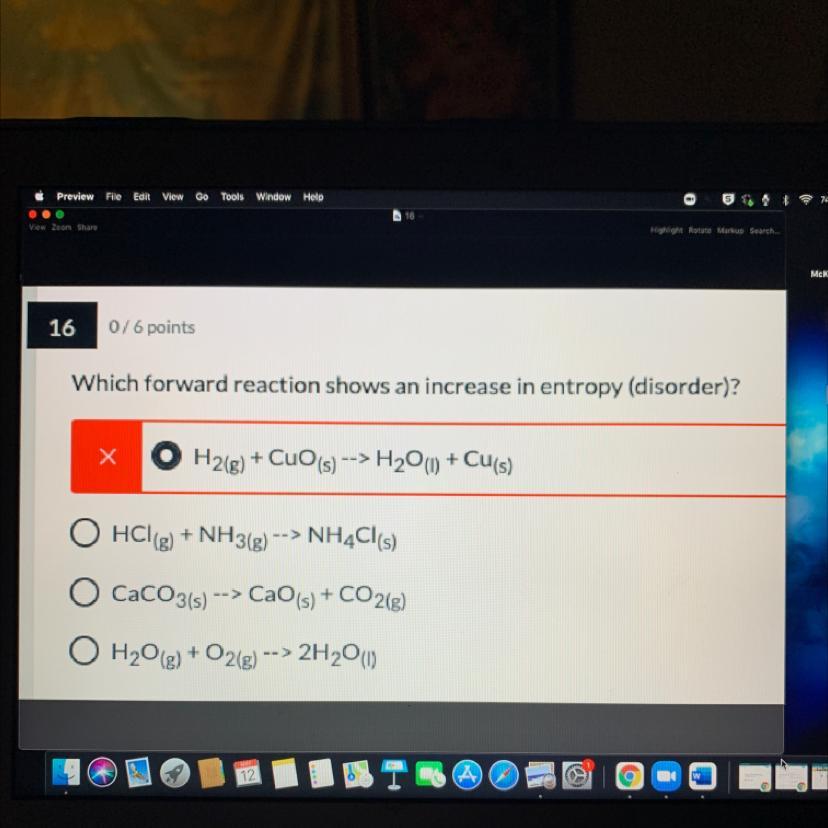
Which forward reaction shows an increase in entropy (disorder)?explain
A.H2(g) + CuO (s) --> H2O(l) + Cu(s)
B.HCl(g) + NH3(g) --> NH4Cl(s)
C.CaCO3(s) -> CaO(s) + CO2g)
D.H2O(g) + O2(g) --> 2H2O)
Answers
Explanation:
Entropy also increases when solid reactants form liquid products. Entropy increases when a substance is broken up into multiple parts. The process of dissolving increases entropy because the solute particles become separated from one another when a solution is formed. Entropy increases as temperature increases. S = entropy
k_{b} = Boltzmann constant
\ln = natural logarithm
\Omega = number of microscopic configurations
Related Questions
Which are three ways water reaches the atmosphere?
A. sublimation, condensation, transpiration
B. evaporation, radiation, and condensation
C. transpiration, evaporation, and sublimation
Answers
Answer:
C. Transpiration, evaporation, and sublimation.
Explanation:
thank you to the internet.
Where must the moon be in its orbit for the full moon phase to be visible?
A
exactly crossing Earth's orbit of the sun
B
on the side of Earth opposite the sun
C
between Earth and the sun
D
directly above one of Earth's poles
Answers
Answer:
A.EXACTLY CROSSING EARTH'S ORBIT OF THE SUNThe moon must be exactly crossing Earth's orbit of the sun for the full moon phase to be visible.
What is Full moon?This is a situation where the Sun and the Moon are aligned on opposite sides of Earth with a corresponding illumination from the Sun.
It also occurs when the moon crosses the Earth's orbit of the sun and is denoted as option A.
Read more about Full moon here https://brainly.com/question/649027
#SPJ2
PLS HELP FOR FINAL!!!!!!!!!!!!!!
Answers
Answer:
The answer is Ernest Rutherford.
Answer :
Ernest RutherfordHope I helpGood Luck
How many atoms are in 5.9 g of N2?
Answers
Answer:
Number of atoms in 5.9 g of N2 = 1.268 *10^23
Explanation:
Weight of one mole of N2 is 28.0134 g
In one mole of N2, number of atoms = 6.023 *10^23
Number of atoms in 28.0134 g (one mole) of N2 = 6.023 *10^23
Number of atoms in 1 g of N2 = 6.023 *10^23/28.0134
Number of atoms in 5.9 g of N2 = 6.023 *10^23/28.0134 * 5.9
= 1.268 *10^23
U SEE WHAT IT SAYS~~~~~~~~
UwUwUwUwUwUwUwUwUwUwUwUwUwuWuWUWUWUWU
Answers
i n the notatation,5N2 what is the number of nitogen atoms
Answers
Answer:
10 nitrogens
Explanation:
The 2 is the number of nitrogens in a molecule of N2. There are 2 of them.
The 5 is a whole different critter. It tells you that it was part of an equation and it took 5 molecules of Nitrogen to balance the equation. You only see numbers to the right of a molecule when the molecule is in an equation.
5 (2)=10
Write the formula for the following compound
sodium chloride
Answers
Answer:
NaCl
Explanation:
Na is the symbol of sodium. Cl is the symbol of chlorine
Help help please help help!!
Answers
Answer:
I believe the answer is numbers
what type of reaction is Pb + CuCl2 ---> PbCl2 + Cu
Answers
The above reaction is a Single displacement reaction , in which lead (Pb) being more reactive displaces Copper (Cu) from its compound [tex](\mathrm{CuCl_2})[/tex] and forms Lead Chloride [tex](\mathrm{PbCl_2})[/tex].
[tex] \boxed{\mathrm{Pb + CuCl_2 \rightarrow PbCl_2 + Cu}}[/tex]
_____________________________
[tex]\mathrm{ \#TeeNForeveR} \: ☃[/tex]
what is the balanced equation of these two
(b) Mn2+ + 2 e- → Mn ℰ° = −1.18 V
Fe3+ + 3 e- → Fe ℰ° = −0.036 V
Answers
Answer:
(b) Mn2+ + 2 e- →2 Mn ℰ° = −1.18 V
Fe3+ + 3 e- → 3Fe ℰ° = −0.036 V
Un átomo neutro tiene número atómico 1 y número másico 3. Indica cuántos protones, neutrones y electrones tiene.
Answers
Answer:
El átomo podes 1 protón, 1 electrón y 2 neutrones.
Explanation:
Cada elemento químico se caracteriza por el número de protones de su núcleo, que se denomina número atómico Z. Es decir, el número atómico de un elemento químico es el número total de protones que tiene cada átomo de ese elemento.
Los protones poseen carga positiva y se encuentran en el núcleo, en torno al cual se mueven otras partículas con carga eléctrica negativa que son los electrones. Así, el átomo es eléctricamente neutro, ya que la carga positiva de los protones está compensada por la carga negativa de los electrones. Entonces, en todo átomo neutro el número de protones del núcleo es igual al de electrones de sus orbitales.
En este caso, un átomo neutro tiene número atómico 1. Entonces la cantidad de protones y electrones presentes en el átomo es 1.
En el núcleo de cada elemento, además de protones, también es posible encontrar neutrones, cuyo número puede variar. La masa atómica (A) se obtiene sumando el número de protones y de neutrones de un núcleo determinado:
Número másico (A) = número de protones + número de neutrones
En este caso, el átomo neutro posee 1 protón y su número másico es 3. Entonces:
3= 1 + número de neutrones
Resolviendo:
3 - 1= número de neutrones
2= número de neutrones
Entonces, el átomo podes 1 protón, 1 electrón y 2 neutrones.
Define An Atom.
Ty!!!
Answers
=> the smallest particle of a chemical element that can exist.
-------☆゚.・。゚ᵴɒƙυᴚᴀ_ƨȶäᴎ❀
newsela what is a chemical reaction
Answers
Answer:
A chemical reaction is a process in which the chemical bonds of a substance are broken or rearranged. One or more substances are formed with different properties because of this chemical reaction. Examples of chemical reactions are rust, combustion, and oxidation.
A 5.0 mole sample of gas has 54 mmHg of pressure at 273K. What is the volume of the gas?
(Round your answer to the correct number of significant figures)
Answers
Answer:
here's the answer hope it helps
Consider the following statements about weeding and identify the incorrect one.
a) Weeding is best done during tilling itself.
b) Weeding is the process of growing weed.
c) Weeding is the process of removal of weeds.
d) Weeding is usually done manually or by using weedicides.
Answers
Answer:
b) Weeding is the process of growing weed
Explanation:
Wedding is an agricultural process carried out to ensure maximum you won't do. It is the process by which weeds i.e. unwanted plants are removed.
As rightly stated in the question;
- Weeding is either done by manual means e.g Cutlasses or use of weedicides, which are chemicals.
- Weeding is best done during tiling operations.
The incorrect option is that "Weeding is the process of growing weed" rather it is a process of removing weed.
Find the number of grams in 16.95 mol hydrogen peroxide (H2O2). Round your
answer to two decimal places and be sure to include the proper units.
Answers
Answer: There are 576.46 number of grams present in 16.95 mol hydrogen peroxide [tex](H_{2}O_{2})[/tex].
Explanation:
Number of moles is defined as the mass of substance divided by its molar mass.
The molar mass of [tex]H_{2}O_{2}[/tex] is 34.01 g/mol. Hence, mass of hydrogen peroxide present in 16.95 moles is calculated as follows.
[tex]Moles = \frac{mass}{molarmass}\\16.95 mol = \frac{mass}{34.01 g/mol}\\mass = 576.46 g[/tex]
Thus, we can conclude that there are 576.46 number of grams present in 16.95 mol hydrogen peroxide [tex](H_{2}O_{2})[/tex].
all metals are minerals
a. true
b. false
Answers
Answer:
B. False is the rite answer
A student measured the pH of his soda and found it to be 4.5.
Calculate the [H3O+] in the soda.
Answers
Answer:
0.0111 M
Explanation:
pH = 4.5
[H3O+] = ?
Relationship between both quantitiesis given by:
pH = -log₁₀ [H3O+]
-pH = log₁₀ [H3O+]
-4.5 = log₁₀ [H3O+]
[H3O+] = e ^ (-4.5)
[H3O+] = 0.0111 M
Copper is a product of the reaction that occurs when dry ammonia is passed over a sample of heated copper(II) oxide. The equation for the reaction is given below. 2NH3 + 3CuO --> 3Cu + 3H2O + N2 Calculate the mass of copper produced if 0.12 dm^3 of nitrogen is produced at room temperature and pressure (rtp). (Relative atomic mass of Cu = 64; One mole of gas occupies 24 dm3 at rtp
Answers
Answer:
0.12 dm^3 x (1 mol/24 dm^3) = ? mols N2
mols Cu = ? mols N2 x (3 mols Cu/1 mol N2) = ?
Then g Cu = mols Cu x atomic mass Cu = ?
2. what is the factor in an experiment that a scientist wants to observe, which may change in response to
the manipulated variable; also known as a dependent variable
Answers
_____Is a non renewable fuel created from the remains of living organisms.
Electricity
Biofuel
Fossil fuel
Nuclear energy
Answers
Calculate the volume that 12.5 g of carbon dioxide gas will occupy at a temperature of 45 degrees Celsius and a pressure of 1.20 atm
Answers
Answer:
[tex]V=6.2L[/tex]
Explanation:
Hello there!
In this case, according to the given information, it is possible for us to realize that we need to use the ideal gas equation in order to calculate the volume of CO2 but firstly calculating the moles:
[tex]n=\frac{12.5g}{44.01g/mol}=0.284mol[/tex]
Then, we proceed with the ideal gas equation to solve for volume:
[tex]PV=nRT=\\\\V=\frac{nRT}{P}\\\\V=\frac{0.284mol*0.08206\frac{atm*L}{mol*K}*(45+273.15)K}{1.20atm}\\\\V=6.2L[/tex]
Regards!
Will lithium become a cation or anion?
Answers
Answer:
Lithium Cation is a monovalent cation that is metabolized much like sodium and is important in many cellular functions inside or on the surface of cells. lithium has a single valence electron that is easily given up to form a cation. Because of this, lithium is a good conductor of heat and electricity as well as a highly reactive element, though it is the least reactive of the alkali metals
Does melting sea ice in the Arctic increase sea level directly? Why or why not? How would melting over Antarctica be different?
Answers
Explanation:
The ice melting would make more water because ice is water and if it melts it make water.
hope this helps :)
Balancee las ecuaciones químicas por el método de tanteo e identifique que tipo de reacción es: 1.1.- Cloruro férrico acuoso reacciona con carbonato de sodio sólido para formar carbonato férrico sólido y cloruro de sodio acuoso.
Answers
Respuesta:
2 FeCl₃(aq) + 3 Na₂CO₃(s) ⇒ Fe₂(CO₃)₃(s) + 6 NaCl(aq)
Explicación:
Consideremos la ecuación no balanceada que ocurre cuando cloruro férrico acuoso reacciona con carbonato de sodio sólido para formar carbonato férrico sólido y cloruro de sodio acuoso. Esta es una reacción de doble desplazamiento.
FeCl₃(aq) + Na₂CO₃(s) ⇒ Fe₂(CO₃)₃(s) + NaCl(aq)
Vamos a usar el método de tanteo. Empezaremos balanceando los átomos de C, multiplicando Na₂CO₃ por 3.
FeCl₃(aq) + 3 Na₂CO₃(s) ⇒ Fe₂(CO₃)₃(s) + NaCl(aq)
Luego, balancearemos los átomos de Fe, multiplicando FeCl₃ por 2.
2 FeCl₃(aq) + 3 Na₂CO₃(s) ⇒ Fe₂(CO₃)₃(s) + NaCl(aq)
Finalmente, obtendremos la ecuación balanceada, multiplicando NaCl por 6.
2 FeCl₃(aq) + 3 Na₂CO₃(s) ⇒ Fe₂(CO₃)₃(s) + 6 NaCl(aq)
(This is rly for science but there’s no category for that) Which statement best describes how scientists and engineers work together
in the research and development cycle?
O A. Engineers come up with scientific questions when they are
developing their design, and scientists do research to answer
them.
O B. Scientists test designs made by engineers and then use the
results to improve the designs.
C. Scientists develop a new technology, and then engineers test it by
doing experiments.
D. Engineers make a scientific discovery, and then scientists perform
research to verify it.
Answers
Answer:
A. Engineers come up with scientific questions when they are developing their design, and scientists do research to answer them.
How many atoms are in 2.2 moles of Zinc?
Answers
what is an element in science
Answers
Answer: Chemical element, also called element, any substance that cannot be decomposed into simpler substances by ordinary chemical processes. Elements are the fundamental materials of which all matter is composed.
Explanation: I hope that was helpful!
How does heart failure affect the lungs?
o Carbon dioxide enters the lungs.
Too much oxygen enters the lungs.
Blood leaves the lungs.
o Fluid can build up in the lungs.
Answers
Answer:
Pulmonary oedema can be caused by lung disease, but when heart failure is more serious, the pressure of blood in the lungs builds-up, pushing fluid into the air sacs. This is how heart failure can lead to respiratory failure. People with pulmonary oedema will feel breathless, weak and unwell.
Measurements of half-life make radioactive isotopes useful for