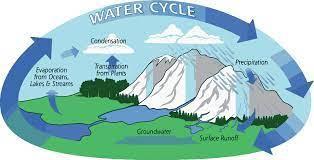
write a paragraph to explain how the transformation of water from one state of matter to another is important for the water cycle

Answers
In order for us to have water to drink, it first needs to go through a process called the water cycle. And in that water cycle there’s a process called evaporation. Evaporation is the process by which water changes from a liquid to a gas. In order for it to rain we need clouds and clouds are made of water. The water is heated causing water molecules to convert into gas, that gas then goes through a process called condensation creating clouds. At some point those clouds will be full of water, and all that water will then cause the cloud to release the water molecules causing rain.
The water cycle shows the continuous movement of water within the Earth and atmosphere. It is a complex system that includes many different processes.
Liquid water evaporates into water vapor, condenses to form clouds, and precipitates back to earth in the form of rain and snow.There are four main parts to the water cycle:
Evaporation, Convection, Precipitation and Collection.
Evaporation is when the sun heats up water in rivers or lakes or the ocean and turns it into vapor or steam.
The water vapor or steam leaves the river, lake or ocean and goes into the air.
Learn more:
brainly.com/question/25226746
Related Questions
5. A sample of an unknown metal has a mass of 120.4 g. As the sample cools from 90.5°C to 25.7°C, it releases 7020. J of energy. What is the specific heat of the sample? Identify the metal among those in the data table below. Finally, is this endothermic or exothermic?
Answers
Answer: The specific heat capacity of the sample is [tex]0.899 J/g^{o}C[/tex] and as heat is released in this reaction so it is exothermic in nature.
Explanation:
Given: Mass = 120.4 g
Heat energy released = -7020 J
Initial temperature = [tex]90.5^{o}C[/tex]
Final temperature = [tex]25.7^{o}C[/tex]
Formula used is as follows.
[tex]q = m \times C \times (T_{2} - T_{1})[/tex]
where,
q = heat energy
m = mass of substance
C = specific heat capacity
[tex]T_{1}[/tex] = initial temperature
[tex]T_{2}[/tex] = final temperature
Substitute the values into above formula as follows.
[tex]q = m \times C \times (T_{2} - T_{1})\\-7020 J = 120.4 g \times C \times (90.5 - 25.7)^{o}C\\C = \frac{-7020 J}{120.4 \times (-64.8^{o}C)}\\= \frac{7020 J}{7801.92} J/g^{o}C\\= 0.899 J/g^{o}C[/tex]
When heat is released in a process or reaction then it means it is exothermic in nature.
Thus, we can conclude that the specific heat capacity of the sample is [tex]0.899 J/g^{o}C[/tex] and as heat is released in this reaction so it is exothermic in nature.
3. a. How many moles of NaCl are dissolved in 152 mL of a solution if the concentration of the solution is 0.364 M?
Answers
Answer:
Good luck man
Explanation:
Good luck man
Which of these elements will successfully create a 1:1 bond with Boron (B)
Phosphorus (P)
Helium (He)
Nitrogen (N)
Aluminum (AI)
Answers
Answer:
Option D, Aluminum
Explanation:
Valency of boron and hence it requires three electron to complete its outermost shell.
Valency of Aluminum is also three and it can donate three electrons to boron to complete the outermost shell of itself as well as the boron.
Hence, Aluminum and Boron will form 1:1 bond with each other. (Al-B)
Hence, option D is correct
during prophase a group of fibers called what forms the cell
Answers
Answer:
During prophase, the nucleus disappears, spindle fibers form, and DNA condenses into chromosomes ( sister chromatids ). During metaphase, the sister chromatids align along the equator of the cell by attaching their centromeres to the spindle fibers.
Explanation:
If I beat it, I ain't wearin' a johnny (Hah)
Adeola wanna roll with a geezer (With a geez)
Is it me or the lifestyle, sweetheart?
Actually, I don't give a shi (Nah)
I'm a rapper now, might as well live in it (Live in it)
Please heeelp to solve these problems
Answers
Answer:
Q.89
Alkane - CnH(2n+2)
given that 8 H = > 8= 2n+2
therefore n= 3
C3H8 = 12×3 + 8×1= 36 +8 = 44
Hikers have noticed that a sealed bag of potato chips puffs up when taken to the top of a mountain. Suppose that at the valley floor below, the air pressure is 1.0 atm, temperature is 25°C, and the volume of the chip bag is 0.985 L. At the top of the mountain, the temperature is 22°C and the chip bag has puffed up to 1.030 L.
What is the air pressure on top of the mountain?
__ atm
Answers
Answer:
0.95
Explanation:
trust
I will give the brainliest
The concentration of a saturated solution of calcium carbonate, CaCO3, is 8.7 × 10-9 Molar. What is the Ksp of calcium carbonate?
A.
4.7 × 10-13
B.
9.3 × 10-5
C.
7.6 × 10-17
D.
8.7 × 10-17
Answers
Answer:
C
Explanation:
x = 8.70 x 10^-9 M
Ksp = [x][x] = x² = (8.70 x 10^-9)^2 = 7.57 x 10^-17
The VSEPR model is used mainly to do what
Answers
Name the following alkene:
CH3CH2CH2CH2CH = CH2
Answers
Answer:
1-hexene. is your answer.
The name of the given alkene is Hex-1-ene
In order to name the given alkene, we will follow some of the IUPAC rules for naming alkenes. The rules are:
1. The longest chain chosen for the root name must include both carbon atoms of the double bond.
2.The root chain must be numbered from the end nearest a double bond carbon atom.
3. The smaller of the two numbers designating the carbon atoms of the double bond is used as the double bond locator.
The given alkene is
CH3CH2CH2CH2CH = CH2
The longest chain has 6 carbon atoms, which means it is a Hexene.
If numbered from the end nearest the double bond carbon atom, the double bond will be on carbon - 1
∴ Hex-1-ene
Hence, the name of the given alkene is Hex-1-ene
Learn more on naming organic compounds here: https://brainly.com/question/24328002
pls help it's about density
Answers
2. 168.55
3. 2.747
So I’m not sure if 2 or 3 are right sorry
what are the effect of water pollition
Answers
a railroad car with a mass of 90,000 kg is traveling along a straight, horizontal track at a constant speed of 2 m/s. the car collides with a second railroad car, also with a of 90,000 kg that is initially at rest. The railroad cars stick together after the collision, as shown in the figure.
1) 0.5 m/s
2) 1 m/s
3) 2 m/s
Answers
Answer:
I think it's 2.
Explanation:
The weight should transfer half of it's velocity to the other one. or its 1
if you traveled 100 miles in 5 hours what is your speed
Answers
Answer:
20 MPH (miles per hour)
Explanation:
You do miles / time (miles divided by time) to calculate the average speed!
Hope this helped ^^
4
If a gas takes up 250 ml at 800. torrand 50.°C, how much space will it take up at 400 torr and 25°?
A 125 ml
B 500 ml
0.46 L
D 0.542 L
Answers
Answer: The space occupied by the gas at 400 torr and [tex]25^{o}C[/tex] is 250 mL.
Explanation:
Given: [tex]V_{1}[/tex] = 250 mL, [tex]P_{1}[/tex] = 800 torr, [tex]T_{1} = 50^{o}C[/tex]
[tex]V_{2} = ?[/tex], [tex]P_{2}[/tex] = 400 torr, [tex]T_{2} = 25^{o}C[/tex]
Formula used is as follows.
[tex]\frac{P_{1}V_{1}}{T_{1}} = \frac{P_{2}V_{2}}{T_{2}}[/tex]
Substitute the values into above formula as follows.
[tex]\frac{P_{1}V_{1}}{T_{1}} = \frac{P_{2}V_{2}}{T_{2}}\\\frac{800 torr \times 250 mL}{50^{o}C} = \frac{400 torr \times V_{2}}{25^{o}C}\\V_{2} = 250 mL[/tex]
Thus, we can conclude that space occupied by the gas at 400 torr and [tex]25^{o}C[/tex] is 250 mL.
The type of stem cells that are thought to be pluripotent are found in
A.) embryos
B.) developed tissues
C.) cord blood
D.) the placenta.
Answers
Answer:
Embryonic stem cells
Explanation:
Answer:
C.)
Explanation:
( Hope this helps 0_0 )
How does genetic variation make a community more stable? Include explanations of how genetic variation is related to the struggle to survive, differential reproductive success, heredity, natural selection, and evolution
Answers
Answer:
If you have genetic variation then a community is more likely to survive because the population is more likely to have individuals who are fine if there are environmental changes. Then natural selection will happen and the species will evolve to he adapted to the new environment. If everyone in a population has the same genetics, than one thing could take them all out.
Why do you think volcanoes are found in a line ?
Answers
Answer:
i never seen a volcano before
Need to finish TODAY ASAP! HIgh point questions
Answers
Answer:
The answer is C. The wire is there so that electrons can flow from one electrode to another, because without the wire, there would be nothing connecting them and the electrons would not be able to flow to each of the electrodes.
calculate the molar mass of hydrogen phosphate
Answers
Answer:
95.9793 g/mol
Explanation:
How does the atmospheric pressure at altitudes above sea level compare with atmospheric pressure at sea level?
A. The atmospheric pressure above sea level is higher.
B. The atmospheric pressure at sea level is higher.
c. The pressures are the same.
D. Differences in pressures cannot be determined.
Answers
Answer:
Option B
The atmospheric pressure at sea level is higher.
Explanation:
Atmospheric pressure can simply be understood as the weight of the air molecules in the atmosphere. The higher we go up into the atmosphere, the thinner the air becomes. This means that the air present at higher heights is not so much, and hence, the air is lighter. This is why most fighter jets and mountain climbers need oxygen tanks to breathe properly.
Due to the fact that the air is less dense (lighter) at higher altitudes, it also means that the atmospheric pressure at higher altitudes is less.
Therefore, the atmospheric pressure at the sea level is higher than the atmospheric pressure at altitudes above sea level
Answer:
B. The atmospheric pressure at sea level is higher.
Explanation:
What is the ion found in a basic solution?
A. OH +
B. H+
C. OH-
D. H-
answer ASAP plz
Answers
10OIS
Save for Later
1
Yusef is doing an experiment to find out how the amount of sunlight affects the growth of a seedling. He has three identical pots
containing grass seeds he planted at the same time. He puts them in windows that receive different amounts of sunlight each day.
Every day. Yusef looks at the pots and records his observations. What is the independent variable in Yusef's experiment?
OA type of soil
OB type of seedling
Ос.
amount of water
OD
amount of sunlight
Reset
Next Question
Answers
Answer:
asdffafAFSAfdsdf
Explanation:
2.3g of lithium chloride in 500cm3 of solution
Answers
Answer:
0.109M
Explanation:
Find molarity of the solution:
Molarity is defined as the ratio between the moles of the solute (Lithium Chloride in this case) per liter of solution. To solve this question we have to find the moles of LiCl and its volume as follows:
Moles LiCl -Molar mass: 42.394g/mol-
2.3g * (1mol / 42.394g) = 0.0543 moles
Volume in liters:
500cm³ * (1L / 1000cm³) = 0.500L
Molarity:
0.0543 moles / 0.500L =
0.109M
I can't do Miscellaneous calculations!
E.g.
How many moles of oxygen atoms are present in 2.5 mole of water molecules?
Answers
take the unknown (the one that the qn is asking for) and ratio it with the known variable
and then put in the mol that you know for the known variable and just do math to get the answer for the unknown
why an offspring and its parent share many common traits?
Answers
Answer:
Explanation: Traits are characteristics, such as its size or height and that comes from parents or other relatives.
What are the 3 most valuable metals in the United States? A. Lead b. Gold c. Iron d.Copper e.Coal f.Silver g.Titanium
Answers
Answer:
Gold, Silver and Titanium
Explanation:
The most valuable metals in the list are:
Gold:
Its value makes it a good investment option. Gold can be used in many different ways, like in jewelry, electronics, medicine, etc.
Silver:
Similar to the case of gold, besides the common value of silver in jewelry, it can be also used in other ways, because it has a large thermal and electrical conductivity.
Finally, the titanium: It's price is actually close to the copper one, but for the cost of work with titanium (Titanium has a really large melting point, then melting it into ingots needs a lot of energy), the price of titanium is increased.
Whch is more acidic, a solution with a pH of 2 or 5
Answers
Answer:
A solution with a pH of 2
Explanation:
The lower a pH is in a liquid, the more acidic it is. 7 is the neutral pH for a liquid. We usually drink around that pH for water. A pH higher than 7 expresses that the liquid is a base.
Answer:
A solution with a pH of 2 is more acidic.
Explanation:
The solution with a pH of 2 is more acidic because on the pH scale as you get closer to zero, the more acidic the solution becomes.
If 10.5 g of iron, at 25°C, absorbs 128 J of heat, what will be the final temperature of metal? (The specific heat of iron is 0.449 J g-1 °C-1)
Answers
Answer:
27.5°C = Final Temperature of the metal
Explanation:
The change in temperature using the specific heat of a material could be obtained using the formula:
Q = m*S*ΔT
Where Q is heat absorbed in Joules = 128J in the problem
m is the mass of the substance = 10.5g
S is Specific Heat of the substance = 0.449J/g°C for Iron
ΔT is change in temperature = Final T - Initial T
Replacing:
128J = 10.5g*0.449J/g°C*ΔT
2.5°C = ΔT
2.5°C = Final T - 25°C
27.5°C = Final Temperature of the metal
A substance has a boiling point of 78 °C. Which of the following is true about the substance? (5 points) a It will also change from a gas to a solid at 78 °C while the gas loses energy. b It will also change from a gas to a solid at 78 °C while the gas gains energy. c It will also change from a gas to a liquid at 78 °C while the gas loses energy. d It will also change from a gas to a liquid at 78 °C while the gas gains energy.
Answers
Answer: If a substance has a boiling point of [tex]78^{o}C[/tex] then it is true that it will also change from a gas to a liquid at 78 °C while the gas loses energy.
Explanation:
The temperature at which vapor pressure of a liquid substance becomes equal to the atmospheric pressure is called boiling point of substance.
At the boiling point, liquid phase and vapor phase remains in equilibrium.
This means that as liquid phase changes into vapor phase and also vapor phase changes into liquid phase at the boiling point.
Thus, we can conclude that if a substance has a boiling point of [tex]78^{o}C[/tex] then it is true that it will also change from a gas to a liquid at 78 °C while the gas loses energy.
Is Zinc sulfide ionic or covalent?